Air pollution in large urban areas is one of the key problems for the protection of the environment and also the well-being of the community. Pollutants are generated by human activity (vehicular traffic, heating, industrial production) and accumulate in the lower parts of the atmosphere in the absence of wind or precipitation.
Since there is increasing concern for the impact of air quality on human health, there is a constant increase in the numbers of manufacturers operating in the construction sector that produce photocatalytic building materials with anti-pollution properties.
Behind the photocatalytic term, that might scare those of you who are not really chemical fanatics (most of us, I would guess), there is a natural process as simple as it is effective.
Photocatalytic process like photosynthesis.
Photocatalysis is a natural phenomenon in which a substance known as photocatalyst uses the ultraviolet light to speed up a chemical process.
To better understand the mechanism of the photocatalysis, we may use the photosynthesis chlorophyll as an analogy.
While plants transform carbon dioxide into oxygen via the photosynthesis chlorophyll process, the photocatalysis transforms pollutants into non-harmful substances by using photocatalytic-based materials such as facade panels, plasters, paving, roof tiles and so on.
The products generated by the oxidation of pollutants by photocatalytic materials are harmless substances, mostly salts, which are present in nature already.
Largely photocatalysis uses titanium dioxide (TiO2), the quintessential photocatalyst. It is a substance that needs ultraviolet light to have a catalytic effect.
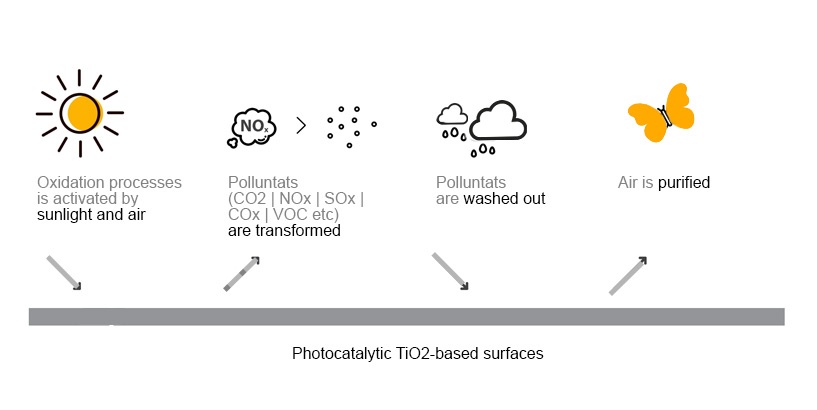
The photocatalysis through ultraviolet rays, and therefore natural solar light or artificial lighting, transforms pollution- such as monoxide and nitrogen dioxide – into nitrate ions and harmless eco-friendly salts, through the production of active oxygen.
Taking advantage of the light energy, the photocatalysts induce the formation of highly oxidizing reagents that are able to decompose by oxidation some organic and inorganic substances present in the atmosphere.
Photocatalysis is therefore an accelerator of oxidation processes that already exist in nature. It favours the faster decomposition of the pollutants avoiding their accumulation.
Main Pollutants in built environment
Among the many organic and inorganic substances present in the atmosphere, the most common three polluting agents are the following:
- Nitrogen dioxide (Nox) develops in the atmosphere from nitrogen oxide, which is primarily produced by the use of fossil fuels such as motor vehicle engines, biomass boilers, etc. According to the World Health Organization, nitrogen dioxide is dangerous to health. A long exposure to nitrogen dioxide can compromise lung functions and increase the risks of respiratory diseases.
- Fine Particles (mainly PM10 and PM2.5) means all airborne powders with an aerodynamic diameter of less than 10 thousandth of a millimetre. This pollutant has the ability to penetrate the respiratory system beyond the larynx. The smaller the size of the particles, the more dangerous they are to human health. The composition of the fine particles varies according to their origins. They may contain sulphates, nitrates, heavy metals or carbonaceous particles.
- Volatile Organic Compound (VOC) is defined by elements belonging to the large category of volatile organic compounds containing carbon, often characterized by pungent odors that are referred to at least a thousand substances. Urban VOC concentrations are almost exclusively produced by the combustion of motor vehicles, coal-fired power stations, incineration pollutants and evaporation of solvents and fuels. From the point of view of health effects, aromatic hydrocarbons are the most dangerous; among simple aromatic hydrocarbons, the most important is benzene. Over longer period of time, VOCs, and in particular benzene, can be responsible for even serious health issues including fatigue, breathing problems, damage to the liver, kidneys, and central nervous system, as reported by the U.S. Environmental Protection Agency (EPA).
Let’s then introduce a little of Chemistry
Titanium dioxide (Tio2) acts as a catalyst in the process of activation and emission of oxygen triggered by the sun’s rays or by a UV lamp. The titanium does not intervene directly in the reaction but favours the photocatalytic reaction by lending its electrons which subsequently regains from the environment.
Photocatalysis generated substances which are harmless to health and the environment.
- Via the degradation of pollutants such as Nox, Cox and Sox, harmless salts such as calcium nitrates, calcium carbonates (limestone) and calcium sulphates are produced respectively
- Carbon dioxide, also called CO2, is turned into a form of carbonate salts;
- Form fine particles, PM10 and PM 2.5, inactive substances, both organic and inorganic, are derived. In addition, the degradation of Nox by photocatalysis also helps reduce the development of PM10;
- Harmless products are generated from the degradation of benzene, even though this process is very slow due to the low reactivity of the oxidants generated by titanium dioxide (TiO2). In nature there are normally many catalysts, so-called “enzymes”, which make possible the chemical reactions passing from the active state to the inactive one recharging continuously, without ever being degraded by the same reactions again.
The secret lies in the oxidative process of photocatalysis of which titanium dioxide (TiO2) has the leading role. In the presence of light (ultraviolet rays), it attracts and retains water molecules, in the form of moisture, which are naturally present in the air. Water molecules capture fine particles and with them, nitrogen oxide. photocatalysis accelerates the oxidation process, that occurs naturally. The photocatalysis drives a faster decomposition of the pollutants.
At this point it seems natural to ask: Since photocatalysis helps to improve air quality, why not apply it to building materials?
The photocatalytic activity has been applied for over a decade to various building materials (glass, ceramics, cement binders) to achieve an “anti-setting” effect.
The worsening level of pollution in urban areas has recently shifted research towards the use of the capacity to reduce harmful substances in the atmosphere.
Actually, there are many titanium dioxides (TiO2) products on the market. Titanium dioxide (TiO2) finds a wide application in the cement industry as well as in interior finishing materials.
Proven application of the photocatalytic principle for cement products show the effective capability of reducing pollutants in the air while providing self-cleaning functions to architectural surfaces. It helps to improve air quality in urban areas and to maintain the aesthetic characteristics of buildings over time.
In cement-based materials, the products of the photo-oxidation reaction are mineralized, due alkaline environment, becoming common inorganic salts such as nitrates, sulphates and calcium carbonates.
These salty substances are partially washed away by rainwater allowing the constant restoration of the initial photocatalytic activity. And the process goes on and on, because it’s activated by solar light.
Cementitious products that exploit the photocatalytic principle are therefore an important contribution to improving the quality of life of urban areas. They also are particularly useful in places where air moment is limited such as tunnels and subways. Artificial light is needed, in the latter, to activate the photocatalytic activity.
Researches undertaken at the University of Ghent (Belgium) demonstrates that photocatalytic surfaces made with TiO2-based products (modified and enhanced) are able to decompose even micro-organisms affecting surfaces, inhibiting the deposition and proliferation of algae, preventing the deterioration of architectural surfaces.
Another interesting feature of a photocatalytic cement-based materials is that they are able to maintain a high solar reflectance feature despite being exposed to dirt caused by car emissions, industrial and domestic combustion processes. This characteristic is particularly useful when applied to building facades. The photocatalytic coating applied to the building envelope reflects the solar irradiation allowing building cooling need reductions. In fact, solar heat is reflected back to atmosphere by the solar reflecting surface (the photocatalytic coating) reducing the heat transfer through the glazing and walls inward resulting in increased internal thermal comfort.
Photocatalytic coatings are also seen as an effective mean to reduce the Urban Heat Island (UHI) effect in densely built areas (this subject will be further explored in another article).
Photocatalytic process can be very useful when applied to indoor finishes such as paint, furniture, tiles along with others materials. These are quite popular and very well used across the global market. Even for these products, titanium dioxide (TiO2) reaction is due to the sunlight that actives the release of active oxygen.
The photocatalytic reaction can also occur through halogen light. For this reason photocatalytic-coated tiles, paints and furniture are also effective in environments such as schools, offices and hospitals. In addition, they offer antibacterial function. As a result, water and common detergents are sufficient for the maintenance of covering surfaces.
Thus, the photocatalytic tiles becomes also an ecological choice, as it allows a significant reduction in the use of detergents needed for cleaning the indoor surfaces. The sole activity of light on TiO2, it is sometimes sufficient to easily clean surfaces.
As for the outdoor surfaces, all the range of indoor pollutants are transformed into salts and carbon dioxide. These salts are deposited on the ground and are removed with the simple daily action of cleaning the indoor spaces. And the process goes on and on, because it’s activated by light. By virtue of this “prodigy” of chemistry, household products have been on the market for some time now.
Titanium dioxide (TiO2) materials act exclusively as catalysts of chemical reactions therefore, just as natural catalysts, titanium dioxide-like “photos” do not exhaust their activity over time, since it is constantly restored by the dynamics of the reaction itself.




